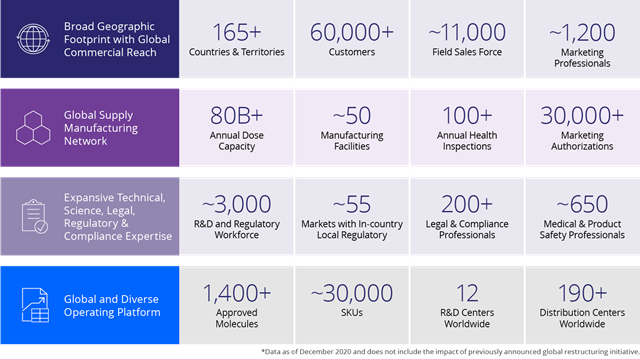
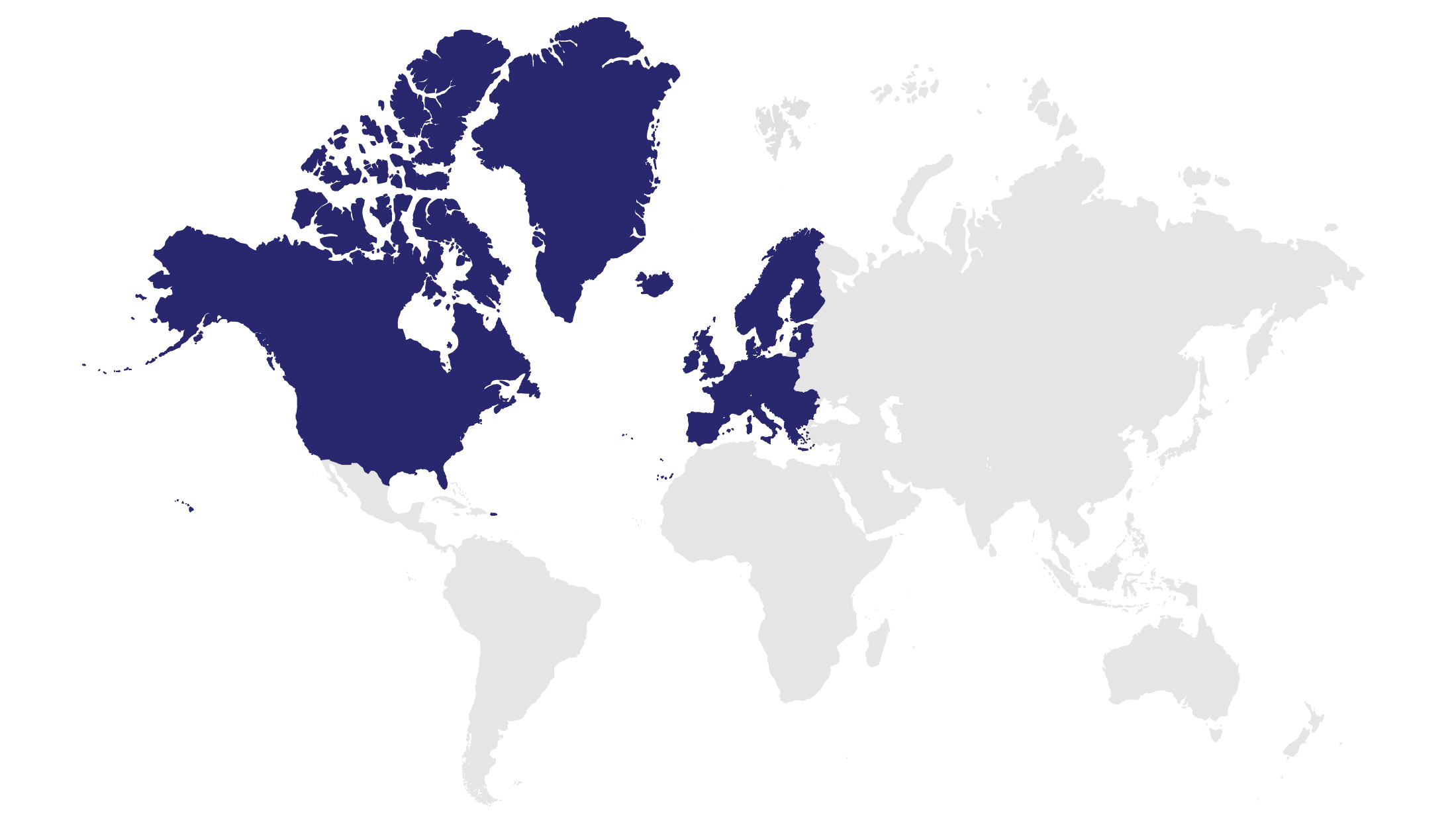
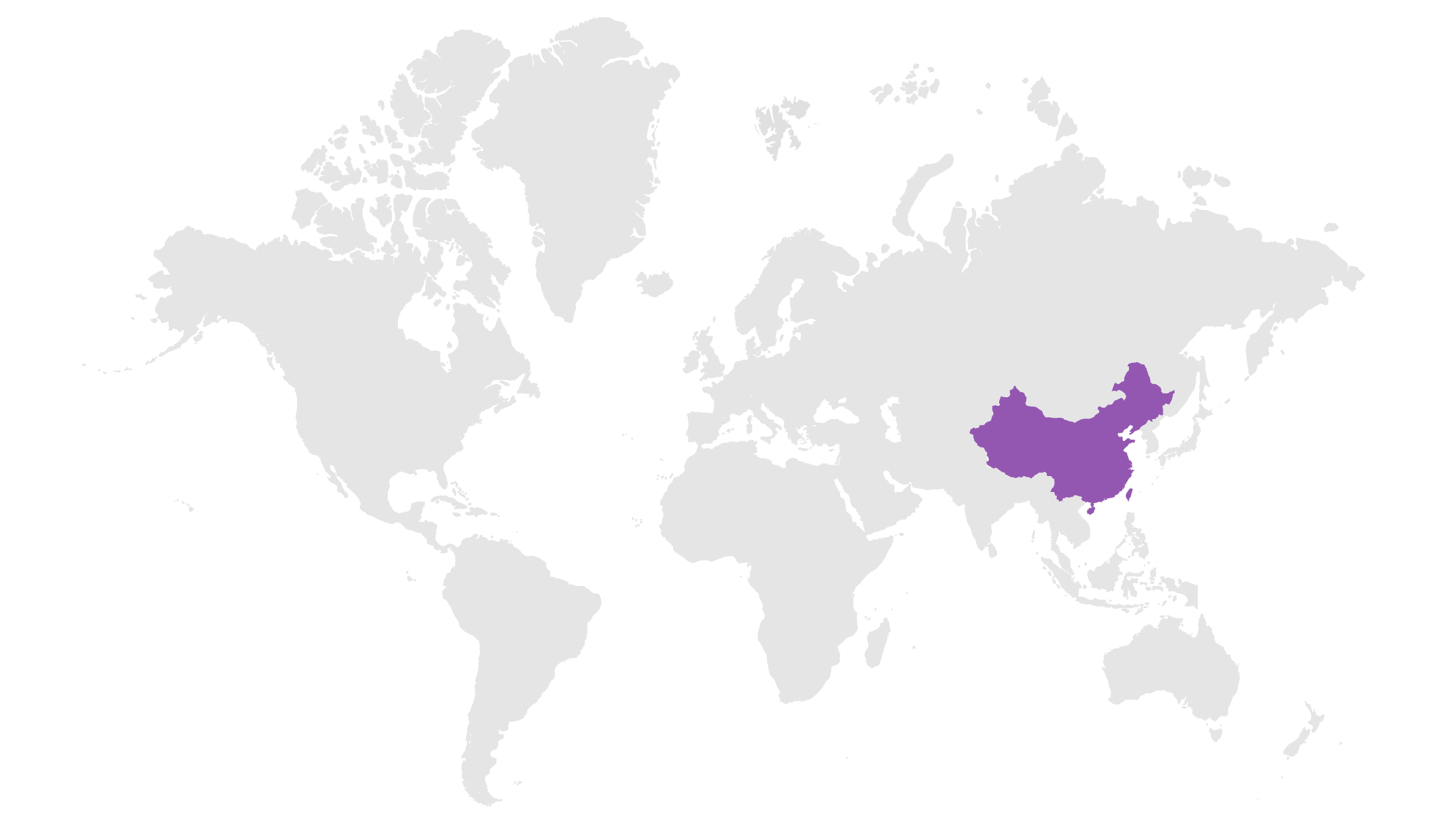
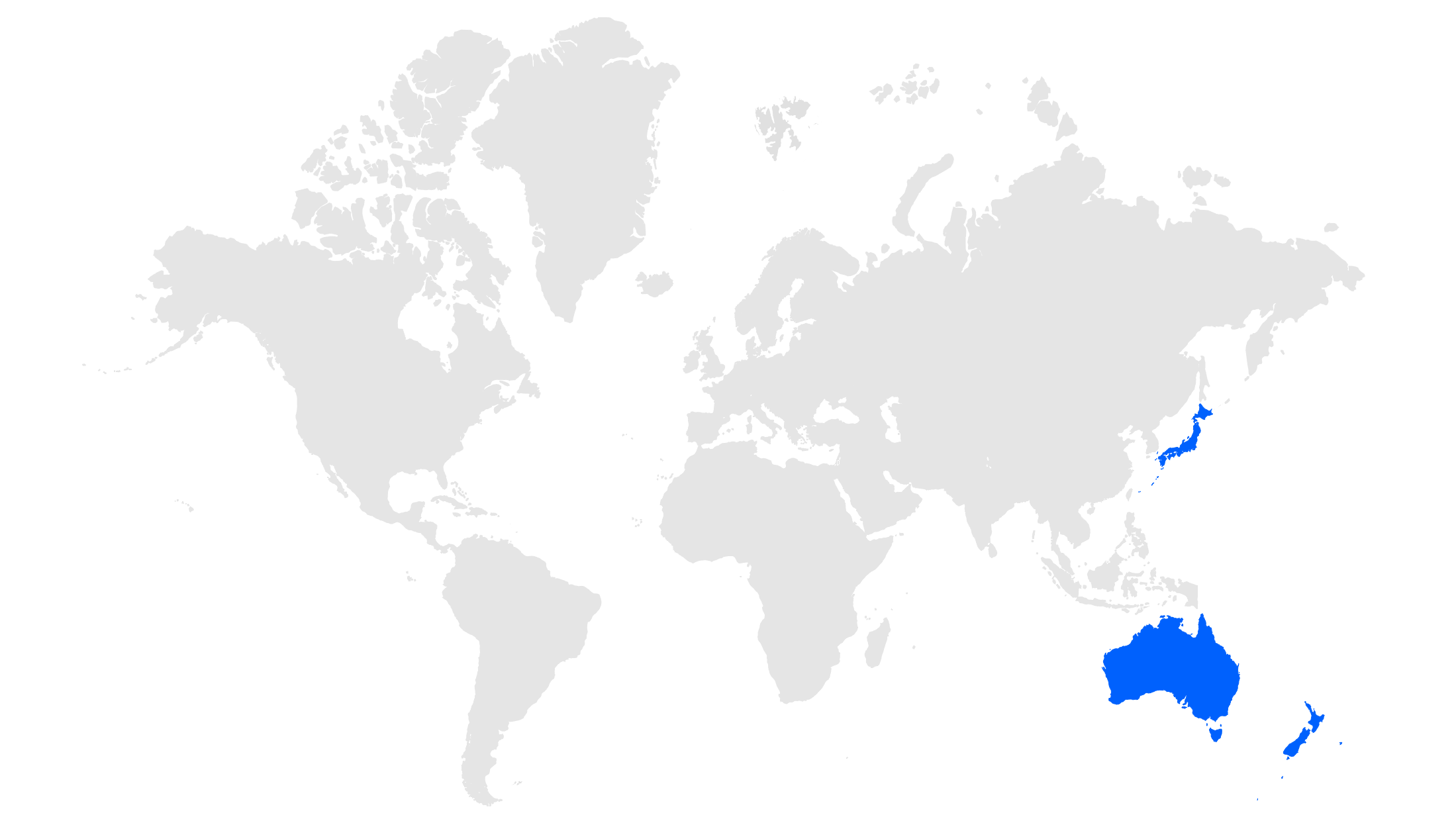
Focusing on innovative, high-growth assets in areas of unmet medical need to drive toward our vision of addressing global healthcare access.

Emerging Markets
Includes over 125 developing countries, such as Brazil, Mexico, Korea, and India
Purpose-fit capabilities

Research and Development
Our research and development capabilities, which are increasingly focused on more complex products, include expertise in formulation, device development, toxicology, analytical, clinical and bioanalytical across a wide range of product types and multiple therapeutic areas.

Regulatory Affairs
Our Regulatory Affairs professionals are based around the globe with a deep understanding of the evolving regulatory landscape and have a successful track record of partnering with other companies and engaging with health authorities to ensure timely and quality regulatory submissions that meet their expectations.

Medical and Clinical Affairs
Our Medical and Clinical Affairs team offers therapeutic area-based expertise to be a trusted partner for healthcare communities and provider of innovative solutions to improve patient health. We also have deep research experience across multiple types of clinical programs in varying phases, product types and patient size ranges.

Manufacturing and Supply Chain
Our extensive global network of manufacturing facilities in geographically diverse locations support our efforts to ensure reliable supply. We have technical capabilities to manufacture across dosage forms and across the complexity spectrum. Our broad distribution network provides flexibility to meet country-specific needs.

Quality
We know that what we do directly impacts the health and well-being of patients. From product development to making or sourcing raw materials to producing finished dosage forms, every step of our development, manufacturing and monitoring processes is grounded in our commitment to good manufacturing practices and the quality and safety of our products.

Legal, Intellectual Property and Compliance
Our experienced legal department provides strong legal support and advice to help expand access to medicine and are deeply committed to operating in compliance with applicable laws and standards.

Sales and Marketing
Our strong commercial infrastructure and reach is driven by sales and marketing professionals around the world with a commitment to improving access and education on trusted, quality medicines. Our extensive commercial experience across brand, complex generics, biosimilars, and generic drugs through various distribution channels enables patient access.

Additional Functional Expertise
We have a range of capabilities and high-performing experts across IT, Finance, Human Relations, Commercial Development, Product Safety, Integration and Business Transformation, and Corporate Affairs, which includes Communications and Corporate Brand, Policy, Government Affairs, Sustainability.